Unit of Concentration Used to Describe Salinity
What is the typical salinity for fresh water. Use the normal concentrations directly ignoring the coefficients in the balnaced chemical equation.

Classification Of Saline Water Salt Farm Foundation
Electrical conductivity EC is also a term used to describe a measurement unit of salinity.
. The concentration of dissolved salts in water. A solution that contains 1 mole of solute per 1 liter of solution 1 molL is called one Molar or 1 M. Electrical conductivity EC is also a term used to describe a measurement unit of salinity.
What unit of measure do we use to describe salinity. You measure the salinity of a seawater sample to be 34 o. EC is measured in units of Deci-Simensmetre or Milli-Simenscm units mmho and is affected by temperature.
Parts per million and parts per billion are used to describe concentrations of highly dilute solutions. Sulfuric acid for example is sold as a 95 aqueous solution or 95 g of H 2 SO 4 per 100 g of solution. Used to describe the concentration of dissolved salts in water the UNESCO Practical Salinity Scale of 1978 PSS78 defines salinity in terms of a conductivity ratio so it is dimensionless.
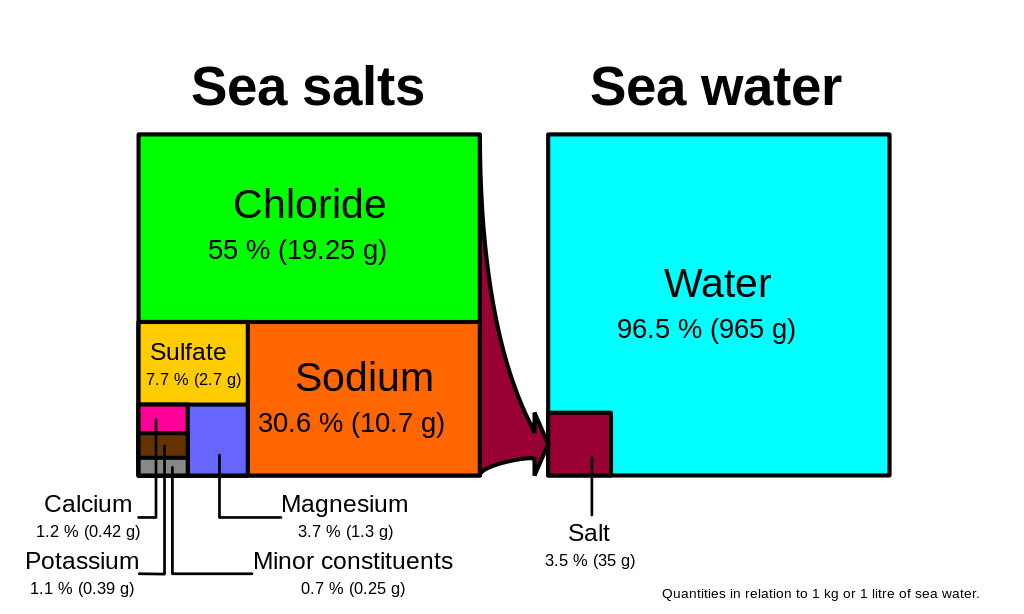
It is usually measured in gL or gkg. On average about 35 g of salt is present in each 1 kg of seawater so we say that the average salinity of the ocean salinity is 35 parts per thousand ppt. The first part of the water cycle in which water liquid changes into water vapor.
Salinity is the saltiness or amount of salt dissolved in a body of water called saline water. Note that 35 ppt is equivalent to 35 parts per hundred. The Practical Salinity Scale of which psu is a component is used to describe the concentration of dissolved salts in water and defines salinity in terms of a conductivity ratio so it is dimensionless.
Salinity is either expressed in grams of salt per kilogram of water or in parts per thousand. The colors of these data indicate the areas of low dark purple to high light yellow salinity in practical salinity units psu. Salts increase the ability of a solution to conduct an electrical current so a high EC value indicates a high salinity level.
The electrical conductivity or EC of a soil or water sample is influenced by the concentration and composition of dissolved salts. 1 molL 1 moldm 3 1 mol dm 3 1 M 1000 molm 3. Salinity is often used to describe seawater and brackish water but it can also be used to describe fresh water and brines.
However molL is a more common unit for molarity. Parts per Thousand ppt is the SI unit for Salinity. Use the appropriate value of K to convert Molarity ie 0250 N H2SO4 0125 M H2SO4 and use the coefficients in the balanced chemical equations to solve for the number of moles of analyte in given sample volume.
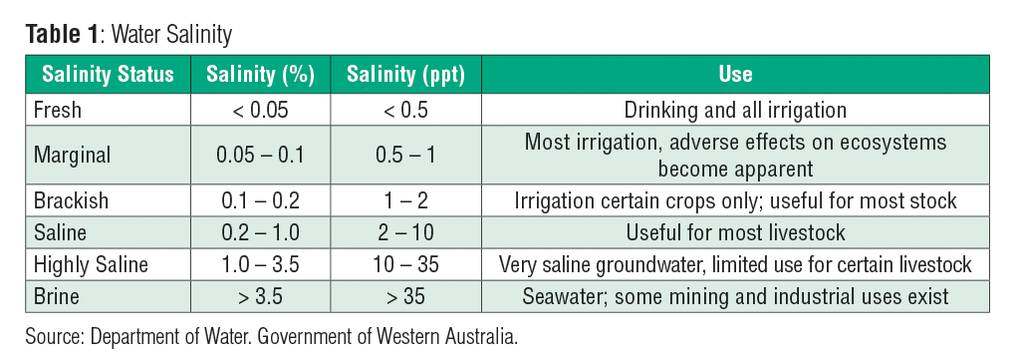
The concentration of dissolved salt in a given volume of water is called salinity. Table 1 includes unit conversions that will be helpful to you as you complete this skill sheet. Some sources now use.
For example ocean water typically has about 35 grams of salt in one kilogram of water so its salinity can be expressed as 351000 or 0035. The higher the salt concentration of the water the greater its electrical conductivity and the lower its osmotic potential. O practical salinity unit PSU.
The average salinity in ocean water you will read in most introduction to oceanography textbooks is 35 ppt. SI stands for International System of Units. The ppm unit can also be used in terms of volumevolume vv instead see example below.
Less than 1 000 What is. In words abbreviation and symbol parts per thousand ppt 000. In words abbreviation and symbol parts per thousand ppt 000.
The unit molL can be converted to molm 3 using the following equation. But in reality hardcore scientists do not use ppt as a unit for the measurement of salinity. Are also referred as electrolytes.
This can also be expressed as 35 or 35 parts per thousand ppt. In general terms the conductivity is a measure of the total ion concentration of the water. The concentration of dissolved salts in water.
Would you describe the concentration of the substance in solution as 2 parts per million or parts per billion. What unit of measure do we use to describe salinity. Much like percentage parts per thousand ppt is a ratio often used to refer to the concentration of solutes in solutions such as salts in water ie.
A solution has 5 grams of a substance in 1. 342 342 ppt 342 342 ppt Density. Salts increase the ability of a solution to conduct an electrical current so a high EC value indicates a high salinity level.
These measurements correspond to milligrams and micrograms of solute per kilogram of solution respectively. The use of conductivity measurements for the determination of salinity was suggested more than 100 years ago by MKnudsen 1903 but until the advent of sophisticated electronic circuitry the method was incapable of achieving the precision and. Salinity was formerly expressed in terms of parts per thousand ppt or by weight parts per thousand or 000.
That is a salinity of 35 ppt meant 35 pounds of. Because both the solute and the solution are both now expressed in terms of grams it could now be said that the solute concentration is 1 part per million ppm. Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it and is a thermodynamic state variable that along with temperature and pressure governs physical characteristics like the.
Salinity was formerly expressed in terms of parts per thousand ppt or by weight parts per thousand or 000. A standard measurement refers to 25C. The SI unit for molar concentration is molm 3.
5 1 ppm 1 mg Solute 1 L Solution. ICP is a suitable option to decrease the salinity of brines with very high salt concentration. The electrical conductivity or EC of water samples is influenced by the concentration and composition of dissolved salts.
PSU to express salinity values where 1 PSU 1 ppt. In an ICP process when ion concentration becomes polarized across the membrane a boundary layer is formed on desalting the surface of the membrane and the salt concentration in the layer is depleted owing to the concentration polarization 63.
Salinity Measurement And Unit Conversion Salinity Management

Representative Ion Concentrations For Standard Seawater High And Low Download Table
Comments
Post a Comment